Valence electrons are the electrons that are present in the last shell. Bromine belongs to halogen family. All the elements in the group have the same valence electronic configuration that is 7. Bromine has an electronic configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Valence shell configuration of bromine is 4s 2 4p5. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Bromine is 35. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. What is Bromine Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is Ar.3d 10.4s 2.4p 5 and the term symbol is 2 P 3/2.
Show the orbital-filling diagram for rm Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy.
Markdown official site. Your only mistake is the order. I'd put 4s2 3d10 4p5, since that is the order of energy of these shells.

Orbital Diagram for Bromine. Bromine has 35 electrons.
Sulfur Electrons
Electron Configuration is 1s22s22p63s23p64s23dp5. Electron Configuration.
An atom's electron. Answer to Show the orbital-filling diagram for (bromine).
Stack the subshells in order of energy, with the lowest-energy sub shell. Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .Show the orbital-filling diagram for Br (bromine).
Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Give the complete ground-state electron configuration for silicon (Si). Nov 14, · Show the orbital-filling diagram for Br (bromine).
Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. I'm having a hard time figuring out the orbial diagramweb.net: Resolved.
1. Describe the two differences between a 2p x orbital and a 3p y orbital.
Chat or rant, adult content, spam, insulting other members, show more. Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more. Show the orbital-filling diagram for Br bromine. Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. I have tried putting this in I I have tried putting this in I don't know how many times, but mastering chemistry keeps telling me its wrong. I'm pretty sure for Br its 3d10 4s2 4p5. Are you sure you want to delete this answer? Your only mistake is the order. I'd put 4s2 3d10 4p5, since that is the order of energy of these shells. Related Questions Show the orbital-filling diagram for Br bromine? Orbital filling diagram for Bromine? Orbital Filling Diagram Chemistry Help!? Show the orbital-filling diagram for nitrogen. Stack the subshells in order of energy, with the lowest-ener? Whats the orbital filling diagram for Br? Answer Questions Chemistry help please:? Equilibrium partial pressure help pls!!? If electrolysis is done on 2. How are metals or materials with high melting points moulded without melting the mould? Do all chemistry teachers know how to make meth? How many molecules are there in 9. Convert grams to ounces? If you blend water in a blender, will the molecules separate? If there arw 9 carat gold jewellery, 18 carat and even 22 carat Why isn't there for example 50 carat gold jewellery, or even carat?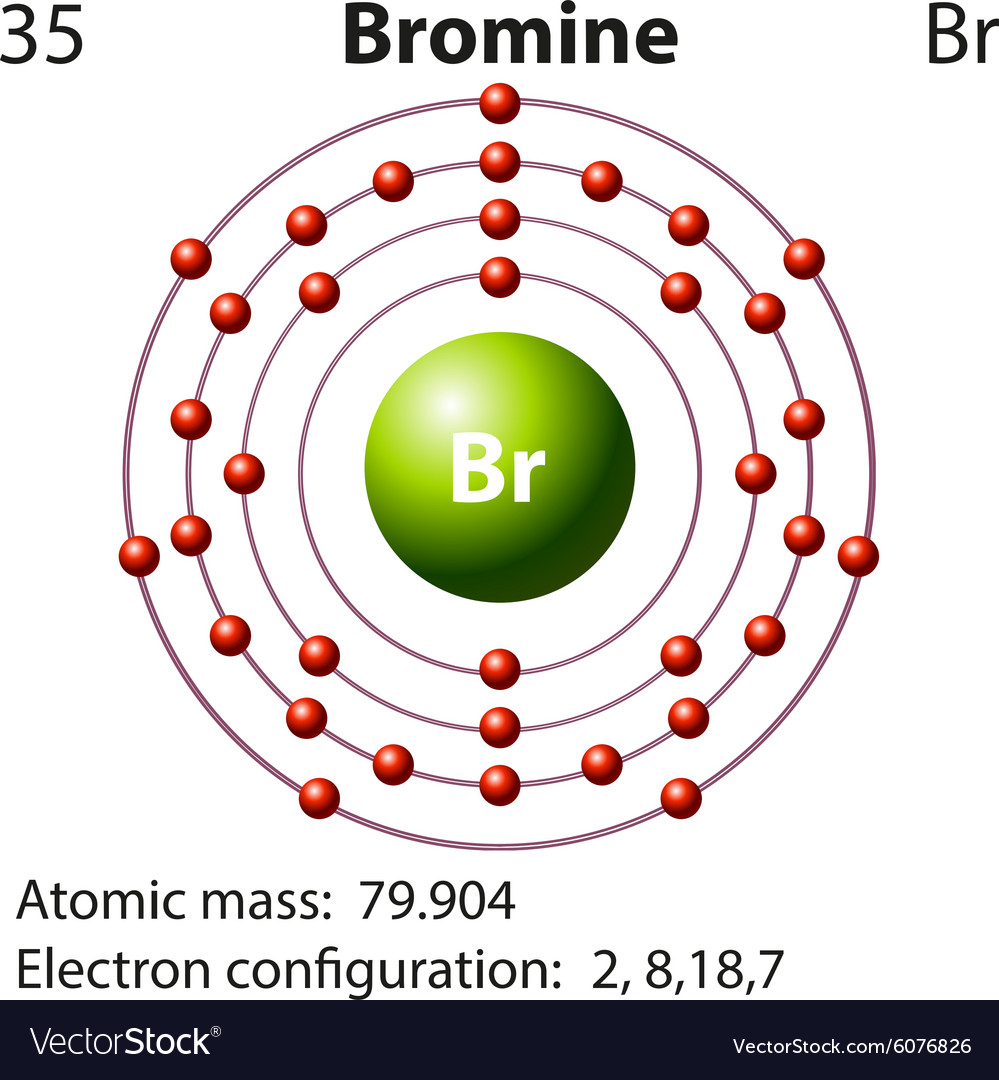
The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital.

2. The lobes of a p orbital disappear at the nucleus.
What does this tell us about electrons in p orbitals? The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth.

Each dot on a circle represents an electron. Periodic Trends.
STUDY. PLAY. Item 1: Part A Show the orbital-filling diagram for N (nitrogen).
Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for Br (bromine).
Stack the subshells in order of energy, with the lowest.Orbital-Filling Diagram for Bromine | Chemistry Learning Apps | Pat ThayerOrbital filling diagram for Bromine?
| Yahoo Answers
Bromine Electrons Gained Or Lost

Click to see full answer
In respect to this, what happens when a bromine ion becomes an ion?
IONS Bromine Can MakeTo become an ion, an element has to gain or loose electrons. If it gains electrons, it receives a negative charge because it then has more electrons than protons. This is known as an anion. If it looses electrons, it receives a positive charge because it has more protons than electrons.
Also, does bromine gain or lose electrons? Bromine atoms tend to gain just one electron to get to a full octet, as Bromine is in Group VII. A chemical consisting of an aluminum ion and a bromide ion in their stable states would be AlBr2+, but it is not an ionic compound because it has a charge. Thus it tends to lose two electrons.
Similarly, will a bromine atom form a positive or negative ion Why?
Bromine Electrons Shell
The neutral atom of bromine has 35 electrons because the number of electrons equals the number of protons. c) Bromine gains an electron, what is the resulting ion called and is it positively or negatively charged? When bromine gains an electron, the resulting ion is called an anion and is negatively charged.
Bromine Electrons Protons And Neutrons
What type of ion does bromine form?
Electrons Protons And Neutrons
A bromide is a chemical compound containing a bromide ion or ligand. This is a bromine atom with an ionic charge of −1 (Br−); for example, in caesium bromide, caesium cations (Cs+) are electrically attracted to bromide anions (Br−) to form the electrically neutral ionic compound CsBr.
